Nuvaxovid
COVID-19 Vaccine recombinant adjuvanted 2. Nuvaxovid dispersion for injection.
After A Decent First Quarter Novavax S Covid Shot Is Struggling
2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US.

. On August 19 2022 the Food and Drug Administration FDA authorized the Novavax COVID-19 Vaccine. On December 20 2021 the. The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix.
This will enable us to start offering the Nuvaxovid. Nuvaxovid-rokote sopii lähes kaikille aikuisille. Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses.
Clinical trials showed that the vaccine has around 90 efficacy. First Approval of the Protein-Based Adjuvanted Nuvaxovid NVX-CoV2373 Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune. This vaccine is currently being used in Sweden and as of date a total of 7000.
Rokotteesta ei myöskään ole haittaa vaikka. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
Beslutet är temporärt och gäller från. Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022.
19 hours agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. 1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Nuvaxovid contains a version of a protein found on the.
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. Name of the medicinal product. 1 day agoSTOCKHOLM Nov.
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Det eftersom att data från Australien gett signaler. Nuvaxovid contains a version of a protein found on the.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. This is a multidose vial.
Like the Novavax vaccine side effects were more. The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix. 1 day agoDet proteinbaserade covid-19-vaccinet Nuvaxovid ska inte ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
The Summary of Product Characteristics is a description of a. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
The addition of the saponin-based. Novavax Announces Shipments Of Its. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. 88 experienced pain. Company Novavax should not be given to individuals younger than 30 the Public Health.
16 fever including 14 severe cases. Qualitative and quantitative composition. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The first batch of Nuvaxovid is expected to arrive in.

Novavax Nvx Cov2373 Nuvaxovid In Europe And Australia Covovax In India And The Philippines Tak 019 In Japan
Novavax Makes One Million Doses Of Nuvaxovid Available For Use In The United Kingdom Pharmtech Focus

Novavax S Covid 19 Vaccine Approved For Canadians 18 And Older Cbc News

Is The Novavax Covid Vaccine Worth The Hype Medpage Today
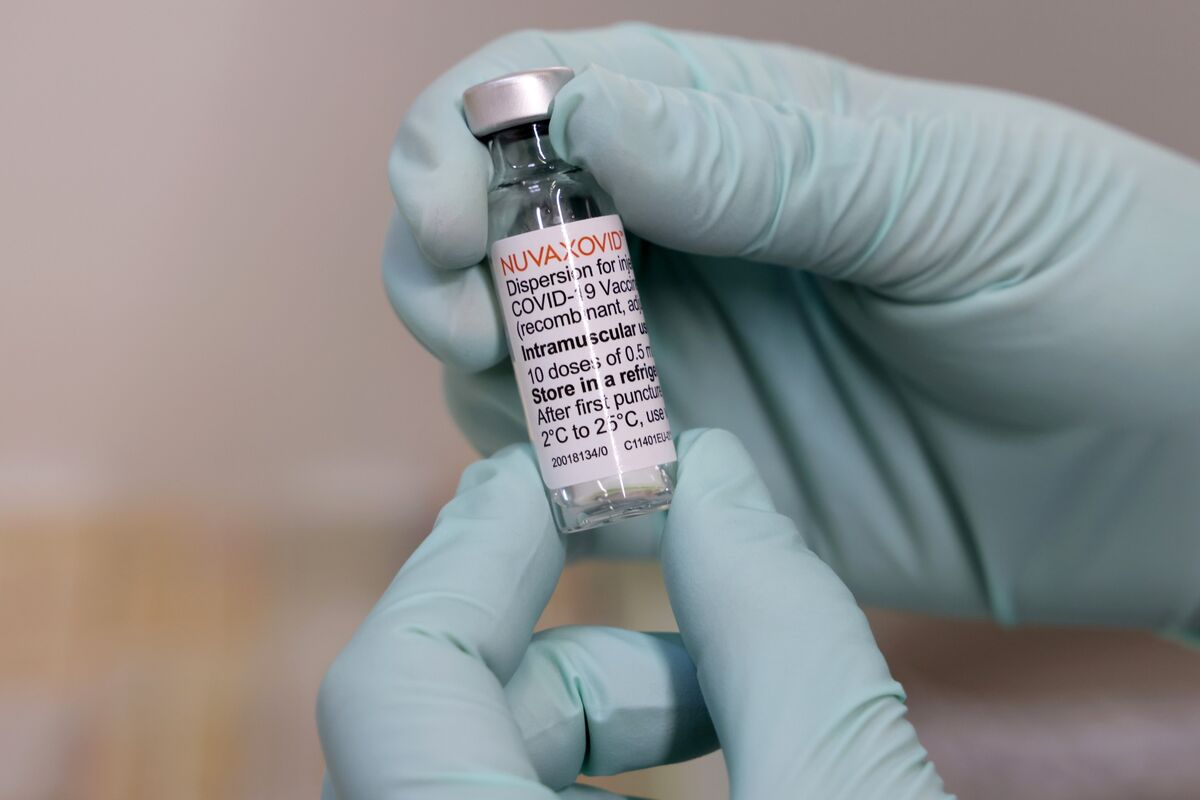
Japan Approves Novavax As 4th Covid Vaccine Amid New Surge Bloomberg

Eu Regulator Backs Use Of Novavax Covid Shot As A Booster

Novavax Says Initial 1m Doses Of Nuvaxovid Covid 19 Vaccine Are Available In Uk

Ec Expands Novavax Nuvaxovid Covid 19 Vaccine Approval
Novavax S Covid 19 Vaccine Moves Closer To Fda Authorization Decision Wsj

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine

Nuvaxovid Novavax Covid 19 معلومات در باره واکسین Australian Government Department Of Health And Aged Care
Nuvaxovid Gets Expanded Provisional Approval In Nz As Covid 19 Booster For Adults
Novavax Nuvaxovid Gets Expanded Conditional Marketing Authorization In Eu For Use As Booster For Adults Aged

Could The Novavax Vaccine Help Us Win This Covid War Medpage Today